My research objective is to develop a fundamental understanding of nanostructure-property-performance relationships of advanced energy materials using multiscale simulation methods (e.g., density functional theory calculations, atomistic/coarse-grained molecular dynamics simulations, continuum modeling, and machine learning) and to build the link between molecular insights and macroscopic properties (e.g., transport, mechanical, chemical, electrochemical, etc.). The molecular understanding of the correlation between physicochemical properties of energy materials can be extended to other functional materials including membranes, colloids, porous materials, biological materials, etc.
Below, I outline some of the projects.
A. Structure-property-performace relationships in novel battery electrolytes including liquid electrolytes and polymer electrolytes
Nanostructure of battery electrolytes, especially the solvation environment of cation, is critical to affecting the property of battery electrolytes and further affecting the performance of related energy storage and conversion devices. For example, the ion pair condition can impact the ionic conductivity and the solvation structure corresponds to the electrochemical stability. High ionic conductivity is needed for supporting the high current density required for fast charging and discharging rates of energy storage devices, and wide electrochemical window will contribute to high energy density of energy storage devices. Therefore, a fundamental understanding of structure-property-performance relationship in many novel electrolytes (e.g., super-concentrated, redox-flow, multivalent, polymer, and ionic liquid-based electrolytes) is highly desired.
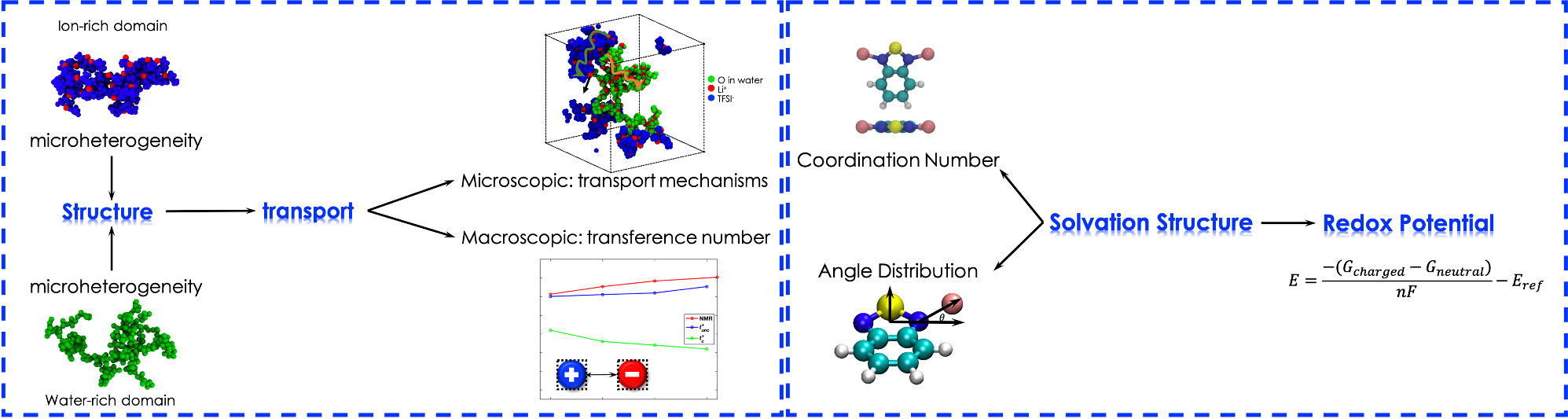
Read about related work at:
a-1. Liquid electrolytes
(1) Mistry, A.; Yu, Z.; Peters, B. L.; Fang, C.; Wang, R.; Curtiss, L. A.; Balsara, N. P.; Cheng, L.; Srinivasan, V.; Toward Bottom-up Understanding of Transport in Concentrated Battery electrolytes, ACS Central Science 2022 (Under review)
(2) Yu, Z.; Balsara, N. P.; Borodin, O.; Gewirth, A. A.; Hahn, N. T.; Maginn, E. J.; Persson, K.; Srinivasan, V.; Toney, M. F.; Xu, K.; Zavadil, K.; Curtiss, L. A.; Cheng, L., Beyond Local Solvation Structure: Nanometric Aggregates in Battery Electrolytes and their Effect on Electrolyte Properties. ACS Energy Letters 2022, 7, 461-470. LINK
(3) Qian, K.#; Yu, Z.#; Liu, Y.; Gosztola, D. J.; Winans, R. E.; Cheng, L.; Li, T., Understanding Fluorine-Free Electrolytes via Small-Angle X-Ray Scattering. Journal of Energy Chemistry 2022, 70, 340-346. LINK
(4) Yu, Z.; Juran, T. R.; Liu, X.; Han, K. S.; Wang, H.; Mueller, K. T.; Li, T.; Curtiss, L. A.; Cheng, L., Solvation Structure and Dynamics of Mg(TFSI)2 Aqueous Electrolyte. Energy & Environmental Materials 2021, 0, 1-10. LINK
(5) Zhao, Y.#; Yu, Z.#; Robertson, L. A.; Zhang, J.; Shi, Z.; Bheemireddy, S. R.; Shkrob, I. A.; Li, T.; Zhang, Z.; Cheng, L.; Zhang, L., Unexpected Electrochemical Behavior of an Anolyte Redoxmer in Flow Battery Electrolytes: Solvating Cations Help to Fight against the Thermodynamic–Kinetic Dilemma. Journal of Materials Chemistry A 2020, 8, 13470-13479. LINK
(6) Yu, Z.; Curtiss, L. A.; Winans, R. E.; Zhang, Y.; Li, T.; Cheng, L., Asymmetric Composition of Ionic Aggregates and the Origin of High Correlated Transference Number in Water-in-Salt Electrolytes. The Journal of Physical Chemistry Letters 2020, 11, 1276-1281. LINK
(7) Hahn, N. T.; Driscoll, D. M.; Yu, Z.; Sterbinsky, G. E.; Cheng, L.; Balasubramanian, M.; Zavadil, K. R., The Influence of Ether Solvent and Anion Coordination on Electrochemical Behavior in Calcium Battery Electrolytes. ACS Applied Energy Materials 2020, 3, 8337-8447. LINK
……
a-2. Polymer electrolytes
(1) Peters, B. L.; Yu, Z.; Redfern, P. C.; Curtiss, L. A.; Cheng, L., Effects of Salt Aggregation in Perfluoroether Electrolytes. Journal of the Electrochemical Society 2022, 169, 020506. LINK
(2) Yu, Z.; Balsara, N. P.; Borodin, O.; Gewirth, A. A.; Hahn, N. T.; Maginn, E. J.; Persson, K.; Srinivasan, V.; Toney, M. F.; Xu, K.; Zavadil, K.; Curtiss, L. A.; Cheng, L., Beyond Local Solvation Structure: Nanometric Aggregates in Battery Electrolytes and their Effect on Electrolyte Properties. ACS Energy Letters 2022, 7, 461-470. LINK
(3) Wang, Y.; He, Y.; Yu, Z.; Gao, J.; Ten Brinck, S.; Slebodnick, C.; Fahs, G. B.; Zanelotti, C. J.; Hegde, M.; Moore, R. B.; Ensing, B.; Dingemans, T. J.; Qiao, R.; Madsen, L. A., Double Helical Conformation and Extreme Rigidity in a Rodlike Polyelectrolyte. Nature Communications 2019, 10, 1-8. LINK
(4) Yu, Z.; Yang, F.; Dai, S.; Qiao, R., Structure and Dynamics of Polymeric Canopies in Nanoscale Ionic Materials: An Electrical Double Layer Perspective. Scientific Reports 2018, 8, 1-11. LINK
(5) Yu, Z.; Fang, C.; Huang, J.; Sumpter, B. G.; Qiao, R., Molecular Structure and Dynamics of Interfacial Polymerized Ionic Liquids. The Journal of Physical Chemistry C 2018, 122, 22494-22503. LINK
(6) Yu, Z.; He, Y.; Wang, Y.; Madsen, L. A.; Qiao, R., Molecular Structure and Dynamics of Ionic Liquids in a Rigid-Rod Polyanion-Based Ion Gel. Langmuir 2017, 33, 322-331. LINK
……
B. Interfacial and transport phenomena involved in energy storage and conversion process
Interfacial properties play an important role in the performance of the energy storage and conversion facilities including batteries and supercapacitors. The study of the interface between electrolyte and electrode can be divided into two aspects. Physically, the classical theories for electric double layers (EDLs) in dilute electrolytes and high-temperature molten salts might not accurately describe EDLs at the interface between electrolytes with nanometric ionic aggregates and strongly electriofied surfaces due to the ionic volume exclusion. Chemically, the interface between the electrolyte and electrode is where the actual electrochemical reaction occur. The solid electrolyte interphases formed at the interface have been considered as an indispensable component to stabilize battery cycling. Nevertheless, the composition, structure, and formation mechansim of the resultant SEI haven’t been understood completely.
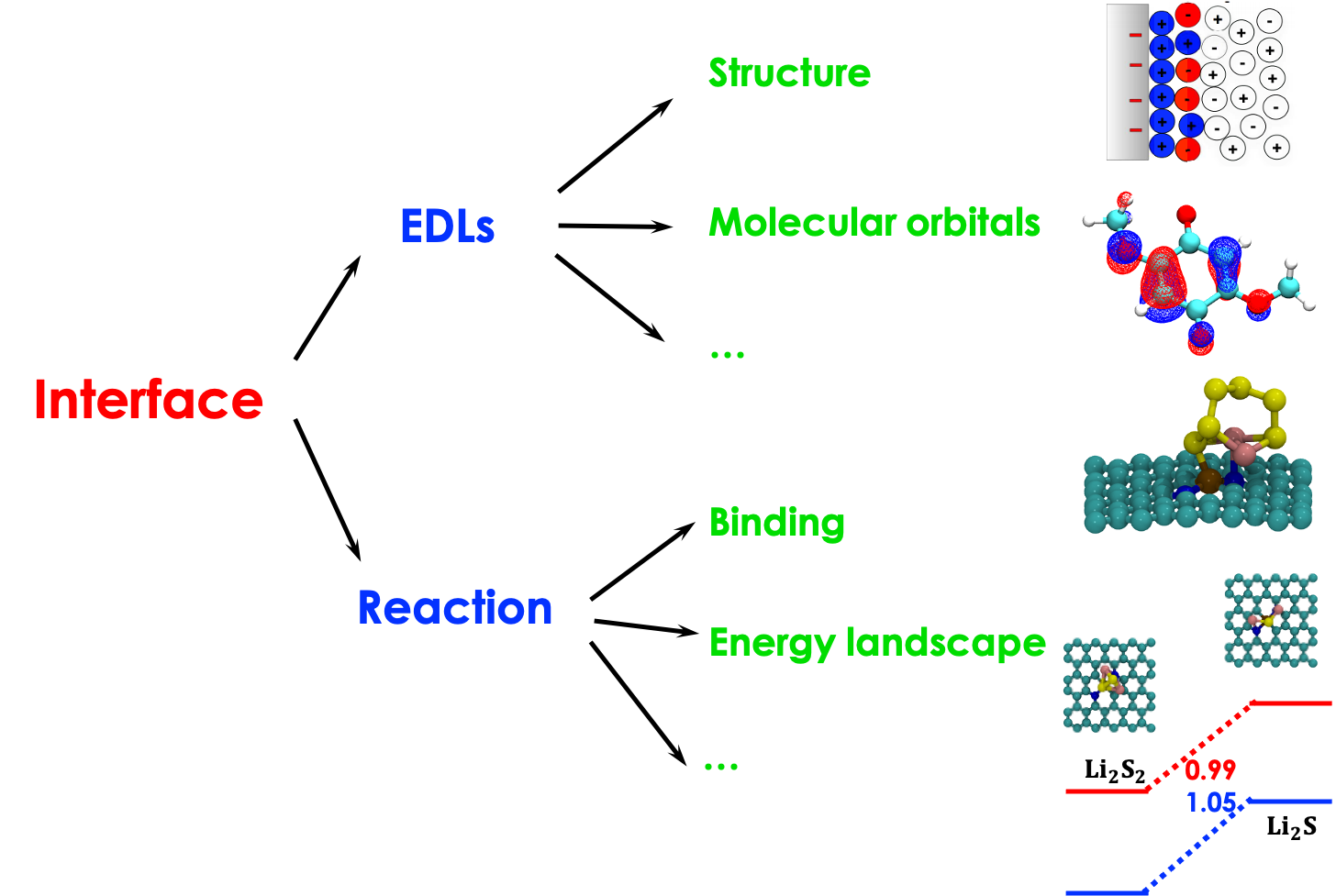
Read about related work at:
b-1. Physically
(1) Wen, X.; Yu, Z.; Zhao, Y.; Zhang, J.; Qiao, R.; Cheng, L.; Ban, C.; Guo, J., Enabling Magnesium Anodes by Tuning the Electrode/Electrolyte Interfacial Structure. ACS Applied Materials & Interfaces 2021, 13, 52461-52468. LINK
(2) Yu, Z.; Yang, F.; Dai, S.; Qiao, R., Structure and Dynamics of Polymeric Canopies in Nanoscale Ionic Materials: An Electrical Double Layer Perspective. Scientific Reports 2018, 8, 1-11. LINK
(3) Yu, Z.; Fang, C.; Huang, J.; Sumpter, B. G.; Qiao, R., Solvate Ionic Liquids at Electrified Interfaces. ACS Applied Materials & Interfaces 2018, 10, 32151-32161. LINK
(4) Yu, Z.; Fang, C.; Huang, J.; Sumpter, B. G.; Qiao, R., Molecular Structure and Dynamics of Interfacial Polymerized Ionic Liquids. The Journal of Physical Chemistry C 2018, 122, 22494-22503. LINK
(5) Zhang, F.; Yu, Z.; Rondinone, A. J.; Huang, J.; Sumpter, B. G.; Qiao, R., Adsorption of Molecular Nitrogen in Electrical Double Layers near Planar and Atomically Sharp Electrodes. Langmuir 2018, 34, 14552-14561. LINK
(6) Yu, Z.; Zhang, F.; Huang, J.; Sumpter, B. G.; Qiao, R., Ionic Liquids-Mediated Interactions between Nanorods. The Journal of Chemical Physics 2017, 147, 134704. LINK
…
b-2. Chemically
(1) Yu, Z.#; Shi, Z.#; Bheemireddy, S. R.; Qian, K.; Li, T.; Zhang, L.; Cheng, L.; Molecular Designing and Characterizing of Novel Fluorinated Ether Solvent for Li Metal Battery. (In preparation)
(2) Zhao, C.; Xu, G.-L.; Yu, Z.; Zhang, L.; Huang, I.; Mo, Y.-X.; Ren, Y.; Cheng, L.; Sun, C.-J.; Ren, Y.; Zuo, X.; Li, J.-T.; Sun, S.-G.; Amine, K.; Zhao, T., A High-Energy and Long-Cycling Lithium-Sulfur Pouch Cell via a Macroporous Catalytic Cathode with Double-End Binding Sites. Nature Nanotechnology 2021, 16, 166-173. LINK
(3) Jiang, Z.-L.; Xu, G.-L.; Yu, Z.; Zhou, T.-H.; Shi, W.-K.; Luo, C.-S.; Zhou, H.-J.; Chen, L.-B.; Sheng, W.-J.; Zhou, M.; Cheng, L.; Assary, R. S.; Sun, S.-G.; Amine, K.; Sun, H., High Rate and Long Cycle Life in Li-O2 Batteries with Highly Efficient Catalytic Cathode Configured with Co3O4 Nanoflower. Nano Energy 2019, 64, 103896. LINK
…
C. Separation
c-1. Separation of rare earth element using ionic liquids
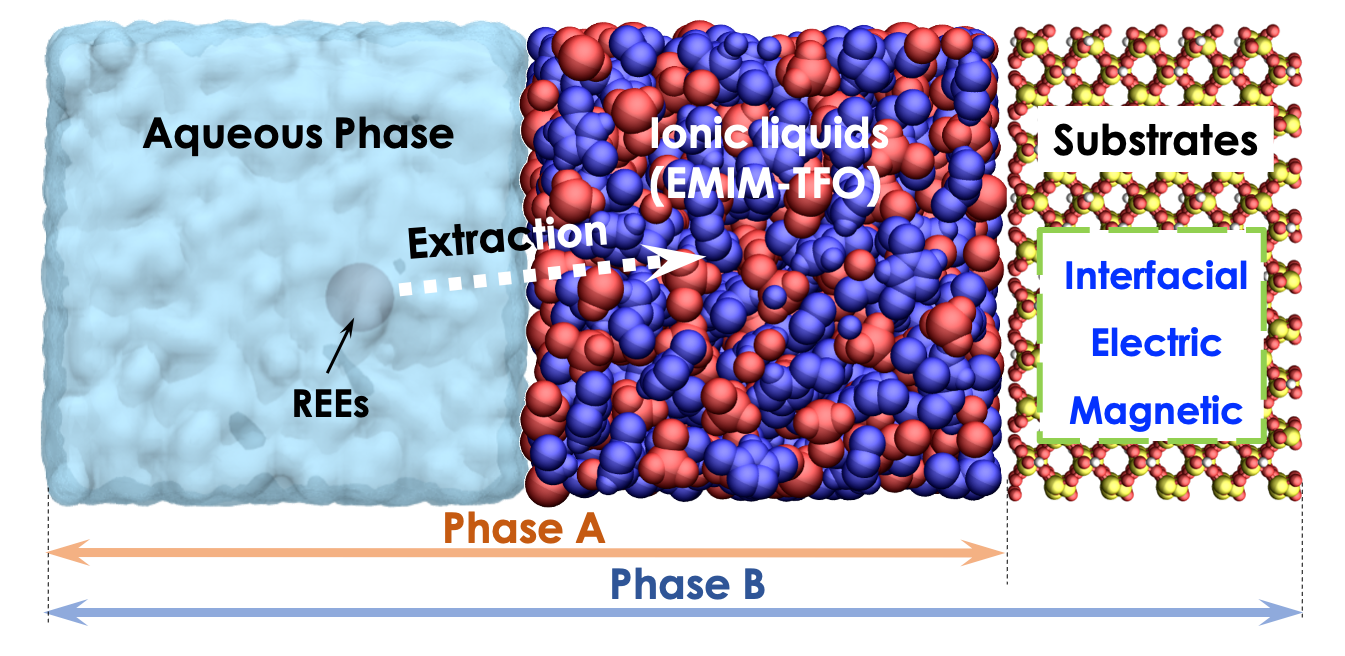
My current work at Los Alamos National Laboratory is focusing on the molecular understanding of rare earth elements (REEs) separation using ionic liquids. REEs are important ingredients for a variety of applications including electronics, catalysts, powerful magnets, etc. Although several seminal studies shed lights on the application of ILs for REEs separations to achieve high extraction efficiency and separation factors, molecular understanding on the extraction mechanism is still in its infancy due to the complexity of the system and the limitations of experimental methods. This project is rationally and progressively prioritized into two phases. Phase A: Molecular understanding of the conventional extraction mechanisms (e.g., anion-exchange, cation-exchange, and solvation mechanism). Phase B: Novel separation schemes induced by interfacial effects, electric fields, and magnetic fields.
c-2. Desalination through GO membranes

I have been involved in a project about the desalination through GO membranes, which elucidated the effects of surface ionization on water transport and salt leakage through graphene oxide membranes. More details are in the following paper.
(1) Fang, C.; Yu, Z.; Qiao, R., Impact of Surface Ionization on Water Transport and Salt Leakage through Graphene Oxide Membranes. The Journal of Physical Chemistry C 2017, 121, 13412-13420. LINK
c-3. Gas-Solid separation

During my master study at Shandong University in China, I have designed and optimized the structure and dimension of a dynamic cyclone to improve the gas-solid separation efficiency using experiments and simulations.
(1) Yu, Z.; Zhao, M.; Ma, C., Experimental and Numerical Investigations of a Dynamic Cyclone. Proceedings of the Institution of Mechanical Engineers, Part A: Journal of Power and Energy 2014, 228, 536-549. LINK